4. Fluid Properties | | 
| | Density Density was already introduced in Equ. 1.1 as the factor relating mass and volume. Since mass is taken as the source of the phenomenon of gravity, and since gravity plays a role in the vertical pressure profile in fluids in a gravitational field, density is—among other things—related to pressure differences in stored fluids or in fluids flowing up or down in pipes. | |
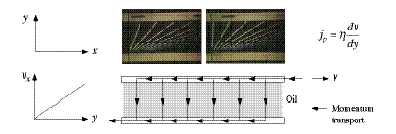
Viscosity Viscosity is the property of fluids that leads to flow resistance. Castor oil, for example, is much more viscous than olive oil, and olive oil is much more viscous than water. Therefore, viscosity critically influences the type of flow. Water flowing out of a tank would normally exhibit turbulent flow. Viscosity is said to be the cause of fluid friction between layers of fluid that move past each other (this is called shear stress). Consider oil between two horizontal plates (Figure 1). If the upper plate is pulled to the right, the layers of oil move at different speeds. The oil sticks to the surfaces of the plates, so the oil speed is highest at the top (equal to the speed of the upper plate) and zero at the bottom. The quantity of motion (momentum) imparted to the upper plate leaves this plate (otherwise it would speed up) and flows down through the layers of oil to the lower plate. The quantity of momentum flowing through the oil every second and per square meter (called the momentum current density jp, unit: Pa) depends upon the gradient of speed in the y direction (dv/dy). The factor relating the transport of momentum and the gradient of speed depends upon how viscous the oil is, and is called the viscosity (eta). | |
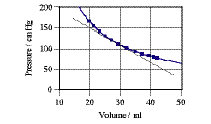
Compressibility and the pressure-volume relation for air Fluids are compressible, meaning their volume changes if the pressure is increased. In general, the compressibility of liquids is low—so we typically assume them to be incompressible. Gases, however, show marked compressibility (see Figure 1). The compressibility of a fluid is defined as the relative change of volume divided by the change of pressure causing the change of volume: 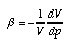 1.11 1.11
The term dV/dp is the inverse of the slope of the tangent to the pV-relation in the pV-diagram (Figure 1). One has to add some more information to the definition such as whether or not the temperature of the fluid was kept constant during compression (which was the case for the data in Figure). For air and other gases at low pressures and high (constant) temperatures, the pV-relation is a simple hyperbola 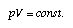 1.12 1.12
if T = const. (T is the temperature). This means that the compressibility of air is equal to 1/p. Air becomes harder to compress if it is already compressed. The constant relating pressure and volume of air at constant temperature depends upon temperature and upon the quantity of the gas used in the experiment: 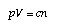 1.13 1.13
n is the amount of substance of the gas, and the factor c depends upon the temperature of the fluid. | | | |