Glycol is heated electrically in an almost perfectly insulated container. An immersion heater, a mixer, and a thermometer are inserted in the liquid, and a well sealed lid is placed on top of the container.
The heater is operated at constant electric power, and the temperature of the glycol is recorded as a function of time (see graph and data file). The power of the electric heater was measured to be 302 W. The mass of glycol is 1.398 kg.
a. Use the graph (print the enlarged pdf) to determine the rate of change of temperature at t = 200 s. b. Formulate the law of balance of entropy for the body of glycol and determine the rate of change of entropy of the glycol at the same point in time (t = 200 s; assume that the glycol is perfectly insulated, and that the mixer does not contribute significantly to entropy production). | | 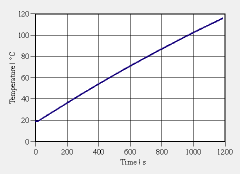
Click to obtain pdf for printing
c. Use the previous results to determine the specific entropy capacitance of glycole at this point in time. d. Repeat the determination of the specific entropy capacitance at t = 400, 600, 800, 1000 s. You should obtain relatively constant results. Discuss your numbers in the light of this expectation. How do assumptions made in your model influence the outcomes? e. Assuming constant entropy capacitance for glycol, determine the temperature as a function of time for heating at constant electric power. (Hint: Formulate the law of balance of entropy, determine the entropy production rate and the rate of change of entropy, and solve the equations). |

