Cold water is prepared (by adding ice to water), poured into an insulated drinking bottle, and left standing in a warm room.
The bottle is places on a magnetic stirrer (there is a magnetic bar inside the bottle to keep the water well mixed at all times). The temperatures of water and of the room are measured as functions of time for 80000 s (see graph). Parameters: Inside diameter of bottle: 7.0 cm, height of bottle: 0.153 m, mass of water: 0.59 kg, thickness of insulation: 6.0 mm, inside heat transfer coefficient: very high, thickness of aluminum: thin, conductivity of aluminum: very high, outside heat transfer coefficient: 12 W/(K·m^2).
a. Explain why the water temperature increases to above that of the environment and then stays there. b. Use the experiment to determine if the temperature of water follows an exponential | | 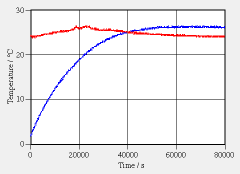
Click to obtain pdf for printing
function (as in the charging of a capacitor). Estimate the time constant of this system (water plus insulated container). If the temperature were to follow an exponential function, what could this mean for the form of a model of the system? c. What is the rate of change of the temperature of water right at the beginning? What is the rate of change of entropy and of energy at this point? d. Formulate the law of balance of entropy and the law of balance of energy for the system made up of water and magnetic bar. e. Calculate the conductivity of the insulation and the power of mixing of water. Treat the insulation as a flat “wall”. |