A spherical piece of aluminum is placed in hot water inside a well insulated glass container. The water is stirred continuously, and there is an insulated lid on the container. Temperatures of water and of the center of the aluminum sphere are taken as functions of time (see diagram). Parameters: Mass of water: 0.636 kg, mass of glass: 0.198 kg, radius of aluminum cylinder: 4.80 cm. Insulation: close to perfect. Specific heat of glass: 800 J/(K·kg).
a. Explain the appearance of the measured temperatures. Sketch the temperature of the metal closer to the surface of the sphere. Sketch the temperature of the glass container (assume the water to have been in the container for some time prior to placing the metal sphere in it). b. From the data taken, determine the initial rates of change of temperature and the final temperature reached by the water and the metal. c. Determine the specific heat and the entropy capacitance of aluminum. d. Use the graph to determine the flows of entropy and of energy out of the water as functions of time. Determine the total entropy and energy transferred. e. Determine the overall entropy and energy conductances for heat transfer from water to metal. Assuming that the convective energy transfer coefficient is about 1500 W/(K·m^2), estimate the thermal conductivity of aluminum. | | 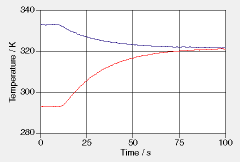
Click to obtain pdf for printing
f. Determine the entropy production rate as a consequence of heat transfer. Use this to find the total entropy produced. Compare this to a direct calculation based on the overall balance of entropy for water and aluminum. Compare the entropy produced to the entropy transferred. Compare the entropy produced in heat transfer to that produced by the magnetic bar stirring the water (power: 1.0 W). g. Imagine the glass to be at room temperature initially. There is no insulation or lid. Hot water is poured in the glass. About 100 s later, the cold metal is placed in the water. Sketch the temperatures of glass, water, and aluminum as functions of time. Remember to allow for heat loss to the environment. h. Sketch a system dynamics model diagram for the system. |
