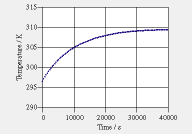
Two experiments are performed with a thick- walled PVC container. Experiment 1: The container is completely insulated from the environment. The container has an initial temperature of 23.4°C. It is filled with hot water having a temperature of 89.5°C. Everything is now sealed, and the water temperature and the temperature at the middle of the container wall are measured (see graph). Experiment 2: Lid and bottom are perfectly insulated. Water and container have an initial temperature of 23.4°C, the same value as the (constant) temperature of the environment. The magnetic mixer dissipates energy at a rate of 2.0 W. Take values of 200 W/(K·m^2) and 13 W/(K·m^2) for the heat transfer coefficients on the inside and the outside of the PVC container. The graph shows the water temperature as a function of time.
Data for the container: Inside radius: 3.0 cm, outer radius: 4.65 cm, inside height: 0.105 m, density: 1400 kg/m^3. a. Use the experiments to determine the specific | | 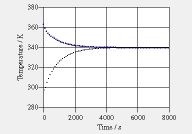
Click to obtain pdf for printing
Click to obtain pdf for printing
heat and the thermal conductivity of PVC. When you have found these values, also determine the specific entropy capacitance and the entropy conductivity. b. Formulate the equations of a dynamical model of the system. Use two storage elements, one for water, the second for the PVC container. Include the effect of the mixer and heat loss through the mantle (lid and bottom are insulated). |


