1. Entropy and Temperature | | 
| | Entropy Thermal phenomena can be understood in terms of entropy and temperature. Entropy is the technical term for what in everyday life we would call heat. It is the substancelike thermal quantity that obeys a law of balance. The properties of entropy are (Fig. 1):
| ¬ | it can be stored in bodies | | ¬ | it can flow into and out of bodies
| | ¬ | it can be produced in irreversible processes (rubbing, burning, electric conduction, absorption of light…)
| | ¬ | it makes bodies warm, or is responsible for melting and evaporation, or it lets air expand…
| | ¬ | it can work, i.e., it can drive other processes and release energy (in heat engines), | | ¬ | or it can be pumped if energy is available (heat pump). | |

Figure 1
|
Temperature Temperature is the measure of hotness, i.e., it tells us how warm a body is. Hotness has a lowest possible value. Therefore we introduce a temperature scale which starts with a value of (absolute temperature scale or Kelvin scale). Temperature is the intensive thermal quantity. Temperature differences serve as the driving force of the flow of entropy (by itself, entropy flows from hotter to colder places; Fig. 2). Temperature is the thermal potential. |

Figure 2 |
Balance of entropy A quantity that cannot be produced (or destroyed) can only flow. Therefore, the quantity stored in a system can only be changed by inflow or outflow. It satisfies the type of law of balance encountered in electricity or fluids: The rate of change of the quantity stored equals the sum of all currents (IS,net, see Fig. 1). Since entropy can be produced (but not destroyed), we must introduce a production rate Pi_S that is included in the law of balance: 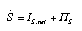 Equ. 3.1 Equ. 3.1
| |
Entropy, temperature, and energy The observation that heat can do work (Fig. 3) establishes the relation between entropy, temperature, and energy. This is in complete analogy to what we have established in hydraulics and in electricity (Chapter 1 and 2, and Energy in Physical Processes). It correpsonds to Carnot's image of the role of heat (caloric –> entropy). |

Figure 3
| | |