3. Measuring Temperature | | 
| | Thermometry (1) Temperature can be determined practically by any substance whose property or properties change if its hotness changes (thermoscopic property). Using such a thermoscopic property, a thermometer and an associated temperature scale can be introduced. Such a scale depends upon the material used in the thermometer. (2) The second challenge of thermometry is to find the value of the lowest possible temperature on the scale introduced in step one. (3) Thermodynamics rest upon a temperature scale which is independent of any material and can be used as an absolute scale. Such an absolute scale is needed to express the relations between entropy, temperature, and energy. | |
Empirical temperatures A practical empirical temperature can be based upon the change of volume of the liquid in a mercury thermometer: Using such a thermometer, we can introduce the Celsius scale: The temperautre of the freezing point of water is set to 0°C, and 100°C is the temperature of vaporization of water at an air pressure of 1 bar. The electric resistance of electric conductors serves as a practical means for determining temperatures. Concrete measurements (Fig. 1, graph on the left) can be approximated by different functions, such as a quadratic one: 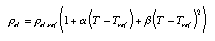 Equ. 3.5 Equ. 3.5
alpha and beta are called the linear and quadratic temperature coefficients, respectively. T_ref is an arbitrary reference temperature. |

Figure 1 |
Thermocouples Thermocouples make use of the thermoelectric effect (as in the Peltier device of Phenomenon 7). The effect consists of a voltage produced as a consequence of a temperature difference. It is observed if two different conducting materials are joined, and the junctions are exposed to different temperatures (Fig. 2, left). Typically, one of the junctions is held at a temperature of 0°C (such as in an ice bath), and the other end measures the desired temperature. The effect, called Seebeck effect, is the result of the coupled flow of electric charge and entropy in the materials. Since the coupling is different for different materials, a voltage develops (Fig. 3). |

Figure 2 
Figure 3 |
Gas thermometers and the model of the ideal gas For a dilute gas, the gas pressure is a linear function of the temperature of a mercury thermometer (Fig. 4, right). For all types of dilute gases the pressure vanishes at the same Celsius temperature of – 273°C (this is the lowest possible temperature). Introduce a new temperature T which is called the (empirical) Kelvin temperature: T = t(°C) + 273 (3.3) Using this scale, p is proportional to T (i.e., p ~ T). Combine this with pV = const for constant temperature and we get 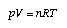 Equ. 3.6 Equ. 3.6
A gas that satisfies this relation is called an ideal gas, and T is also called the ideal gas temperature. n is the amount of substance of the gas, V is the volume, and R = 8.314 J/(K·mole) is the gas constant. The ideal gas temperature can be used as an absolute temperature scale (independent of any material) that measures the thermal potential. |

Figure 4
| | |