4. Temperature-Entropy Characteristic of Simple Materials | |

| |
Adding entropy to a simple fluid In the simplest type of material (water, sandstone, iron…) the temperature simply depends upon how much entropy it contains. More precisely, the temperature of the material is a function of its specific entropy (entropy per mass) only. The relation between specific entropy and temperature must be measured, and it can be represented graphically (Fig. 1), in a table, or by an analytical approximation. Measuring the entropy added to a body is simplest in the case of a liquid such as water since it can be stirred during heating which makes the temperature the same throughout the body. The entropy added can be calculated from the energy flowing from the heater (Fig. 2): IS = IW /T. We introduce the specific entropy of a material as the ratio of the entropy it contains and the mass of the body: 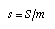 Equ. 3.7 Equ. 3.7
Note that the choice of zero point of the entropy is arbitrary. Mechanical engineers often use 0°C for this purpose, chemists use 25°C. |

Figure 1

Figure 2
|
The warming factor The most significant feature in the Ts-diagram of a material is the slope of the T(s) curve. It tells us how fast the temperature rises as a function of an increase in entropy. This slope is called the warming factor (Fig. 3): 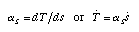 Equ. 3.8 Equ. 3.8
If T(s) is a straight line in the Ts-diagram, the temperature can be calculated easily with the help of the warming factor: 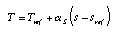 Equ. 3.9 Equ. 3.9
|

Figure 3 |
Entropy capacitance Commonly, the inverse of the warming factor is used to represent the relation between temperature and entropy of a material. The inverse of the heating factor is called the specific entropy capacitance k_S: 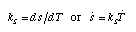 Equ. 3.10 Equ. 3.10
The entropy capacitance, K_S = m·k_S, has the usual meaning of a capacitance, as in hydraulics or electricity. In general, the entropy capacitance depends upon the temperature. The change of entropy of a body can be calculated graphically from the temperature – entropy capacitance diagram (Fig. 4). |

Figure 4 |
Specific heat: The temperature coefficient of energy It is common as well to introduce the specific heat c which is calculated from the specific entropy capacitance by multiplying the latter by the temperature of the material (c = T·k_S). It allows us to determine directly the change of the energy of the simple materials discussed here: 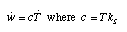 Equ. 3.11 Equ. 3.11
The specific heat of materials generally depends upon the temperature of the material. The change of energy of a body can be determined graphically from the specific heat – temperature diagram (Fig. 5). Note that the meaning of the specific heat is that of the (specific) temperature coefficient of energy (it is not a capacitance!). |

Figure 5
|
Entropy-temperature relation of bodies having constant
temperature coefficient of energy Some materials (water, solids at high temperature) have almost constant specific heats. For these materials, entropy and energy can be calculated easily: 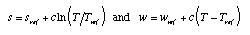 Equ. 3.12 Equ. 3.12
| |