5. Entropy and Energy Transfer in Heating and Cooling | | 
| | Overall entropy tansfer When entropy flows we normally speak of heat transfer. Entropy flow is the result of (1) spontaneous flow through matter due to a temperature difference (Fig. 1,
conductive transport), (2) convective transport with fluids, or (3) radiation. If one or all of these processes lead to a flow through a series of layers from a hot body to a colder body, we speak of overall entropy flow. The entropy flux I_S is expressed in terms of an entropy conductance G_S and the temperature difference 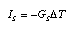 3.13 3.13
Usually, the conductance is written in terms the product of an (average) entropy transfer coefficient hS and the surface A through which the flow takes place: 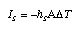 3.14 3.14
|
Figure 1
|
Energy current accompanying the entropy current in heating and cooling The entropy flux through a surface is related to its associated energy current by the temperature T at the surface (Fig. 3.24):  3.15 3.15
G_W is called the overall energy conductance. h = T·h_S is called the overall heat transfer coefficient. | |
Dynamical models Combining this simple expression of an entropy or energy current through a series of layers with the relation of entropy and temperature of a body, and the entropy balance for that body, leads to useful dynamical models for the cooling or heating of uniform bodies (Fig. 2). The simplest models of cooling of bodies making use of constant material properties leads to an exponentially decreasing temperature. |
Figure 2 |
Calculating the energy conductance (or resistance) The energy conductance of a series of layers (Fig. 3) is equal to the inverse of the thermal resistance of this series: 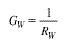 3.16 3.16
which in turn is the sum of the resistances of all the layers: 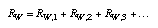 3.17 3.17
The resistance of a layer depends upon its properties and the type of heat flow. The resistance of a flat conductive layer is 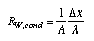 3.18 3.18
lambda is the thermal (energy) conductivity of the material (lambda_S = lambda/T is the entropy conductivity), delta_x is the thickness of the layer, and A is its cross section. Conductive resistances R_cond depend on the geometry of the body conducting heat. Conductive resistances R_cond depend on the geometry of the body conducting heat. Therefore, the expression in Equ. 3.18 is different for container walls such as a thick cylindrical shell or a spherical shell. The resistance of a convective interface between fluids and solids (Fig. 4) is 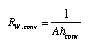 3.19 3.19
Here, hconv is the convective heat transfer coefficient which includes all the interesting and difficult details of this type of transfer which depends upon the characteristics of the flow (for example the speed) of the fluid past the solid surface. |
Figure 3
Figure 4
|
Bodies in thermal contact Heat transfer, i.e., conductive entropy transfer, is irreversible. Entropy is produced always if entropy flows from a hotter to a colder place. This effect has to be taken into account in dynamical models involving more than one body, i.e., as soon as we have two or more bodies in thermal contact whose thermal processes we want to understand. Since entropy production is a consequence of the transfer of heat, and the transfer takes places through transfer layers, we have to modify our model of a thermal resistive element (or elements). The process diagram of a thermal resistor must include a source due to entropy production (Fig. 5 and Fig. 6). The entropy production rate in a thermal resistive element is again calculated from the dissipation rate. The energy dissipated is the energy released by the fall of entropy from T1 to T2 (Fig. 7). The entropy production rate is 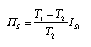 3.20 3.20
A possible representation of entropy production due to entropy transfer in a system dynamics model is the is shown in Fig. 8. The stock in the middle is as a symbol for the entropy of the transfer layer between the bodies. Since the transfer layer is a resistor, it does not store entropy. Therefore, the flow out of this element equals the sum of the flow in and the entropy production rate in the element: 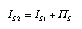 3.21 3.21
|
Figure 5
Figure 6
Figure 7
Figure 8
| | |