6. Heat Engines and Heat Transfer | | 
| | Ideal heat engines and heat pumps When entropy flows through a heat engine from a high to a low temperature (from a burner to a cooler), energy is released (see Figure 2.1). If all the energy released is used to drive the desired process (mechanical, electrical, etc.), the engine is said to be ideal. In this case the process diagram of the engine takes the form shown in Fig. 1 (left). It is customary to introduce two measures of efficiency. The first is the ratio of useful energy current to energy supplied by the burner (called the thermal efficiency or first law efficiency): 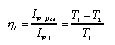 3.22 3.22
the second is the ratio of useful power to thermal power (called the second law efficiency): 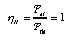 3.23 3.23
For an ideal heat engine (Fig. 1, left), the second law efficiency is equal to 1. For heat pumps (Fig. 1, right) one introduces the coefficient of performance which is the ratio of the useful energy current to the power of the driving process: 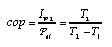 3.24 3.24
For refrigerators, the figure of merit is the ration of the energy current removed from the cold space to the driving power. |

Figure 1
|
Overall irreversibility of a heat engine Heat engines do not work reversibly. There are many different reasons for entropy production. The most important are friction and heat transfer, and among these heat transfer dominates. The total entropy production of a heat engine (or of a thermal power plant) can be determined from measured temperatures energy flows. Consider the process diagram of a heat engine as in Fig. 2. If the temperatures are fixed, the entropy production in the plant leads to a higher entropy outflow than in the reversible case. The stronger entropy current carries with it more energy. Therefore, the useful energy current (I_W,use) has become smaller, the efficiency of the engine is smaller than in the reversible case. If we assume that the temperatures and the real thermal efficiency eta_I,real = IW,use/IW1 can be measured, we can use these quantities to determine the overall entropy production rate of the plant: 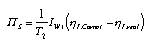 3.25 3.25
|

Figure 2
|
Heat exchangers in thermal engines Heat transfer takes place during heating and cooling of the working fluid. Heat exchangers are added between the furnace and the fluid, and between the fluid and the cooler. The fluid is assumed to operate reversibly. All irreversibilites take place in the heat exchangers (Fig. 3). Such a model engine is called endoreversible. With T1, T2, and IW1 held fixed, the model can be optimized by determining the condition of minimal entropy production rate (which coincides with maximum useful energy current). This leads to the so-called Curzon-Ahlborn efficiency: 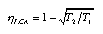 3.26 3.26
|

Figure 3
| | |