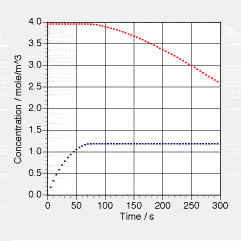
Two communicating containers having an additional outflow are set up exaxtly as in => Problem 1.1 of Chapter 1. Data of volumes of water in the tanks as functions of time given in that problem apply exactly to this problem here. 1.00 g of table salt are dissolved in the larger Tank 2. From the viewpoint of hydraulics, the processes run as described in Problem 1.1. Here we have additional data of the (molar) concentration of salt in the two tanks. Note that the data starts when the flow starts according to Problem 1.1 (there, t0 = 10 s).
a. Determine (or find from tables or the Internet) the molar mass of table salt (NaCl). b. Determine the concentration of salt in Tank 2 at the beginning and compare to the data given in the graph. c. How much water has flowed into the smaller Tank 1 until the levels in the tanks are equal? (t1 = 75 s in the graph on the right; corresponds to t = 85 s in the graph of Problem 1.1). How much salt must have flowed into Tank 1? By how much did the amount of salt change in Tank 1? How much salt is in Tank 1 when the levels are equal? d. Use the results from c to determine the concentration of salt in Tank 1 at the time when both water levels are equal. e. How much salt has flowed out of Tank 2 from t0 = 0 s to t1 = 75 s? How much salt has flowed out into the environment through the second hose? | | f. Explain why the concentration of salt in Tank 1 increases from t0 = 0 s to t1 = 75 s whereas it stays constant in Tank 2 during this phase. g. Explain why the concentration of salt in Tank 1 is constant after t1 = 75 s whereas it deacreases in Tank 2. h. At t = 150 s, determine all currents of amount of salt. Hint: You need the flows of water from Problem 1.1. i. Determine the rates of change of amount of salt for Tank 1 and for Tank 2 from these currents and compare to what you can measure in the graph(s).
Click to obtain pdf for printing
|