There are two anomers of glucose (C6H12O6): alpha-glucose and beta-glucose. When solid alpha-glucose is dissolve in water, part of it slowly transforms into beta-glucose. By optical means one can determine the concentrations of the two types of glucose (see Investigation 5 for more details; data in the graph on the right). Assuming the transformation A <––> B is of first order and linear in the difference of the chemical potentials of the anomers, we can write the differential equation for A as (1) dnA/dt = V·k·(mu_B – mu_A) (2) dnA/dt = V·k'·(c_B – K·c_A) where K is the equilibrium constant, V = 1.5e-5 m^3 the volume of the solution, and k and k' are reaction constants. a. After about 10000 s, the concentrations stay almost constant. What does this mean for the difference of the chemical potentials in this range? What does this mean for the value of the equilibrium constant K? (How big is it?) b. Using the result for K, what is the value for the standard chemical potential difference (the difference for standard values of concentration of 1.0 mole/L)? Which of the potentials is higher at standard conditions (that for alpha-glucose or the one for beta-glucose)? c. Now calculate the chemical potential difference between alpha and beta glucose for | | 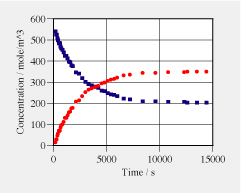
Click to obtain pdf for printing
t = 1000, 2000, 3000, 4000 and 5000 s. Use this to determine the the reaction constant k for these five points. Do you get a (more or less) constant value for k? d. Use the data to determine the reaction constant k' for the second form of the reaction equation. e. Imagine, 0.0050 mole of alpha glucose is quickly mixed into the solution at t = 15000 s. Calculate the new chemical potential difference. Use this to determine the new (initial) rate of change of n_A and n_B. Sketcht the amounts of substance as a function of time. |
