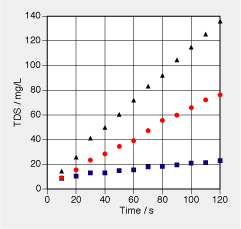
In an experiment, salt water is placed into a length of dialysis tubing which is then closed off at both ends. The tube is placed in fresh water which is gently stirred and whose conductivity is monitored as a function of time. The data reported in the graph is for three different solutions of NaCl in the dialysis tubing: 10 g/L, 50 g/L, and 100 g/L. There are 10 mL of solution in the tube each time. The tube is placed in 300 g of fresh water in a large container.
a. Measure the (average) rate of change of concentration of salt in the large container. Use this to determine the rates of change of amount of salt in the container. b. What was the current of amount of substance of NaCl for the three experiments? c. What form of a constitutive law for the flow of NaCl across the dialysis membrane is suggested by the results in b? d. Determine the conductance for the flow of amount of salt for the three cases. Assume a flow law in the form I_n = - G_n·(c_2 - c_1), where c is the molar concentration of NaCl. e. Estimate the surface area for the dialysis tubing containing the 10 mL of solution. From this estimate the transport coefficient k in j_n = k·(c_2 - c_1) for the transport of salt from one side of the tubing to the other. | | f. Take the data of the second experiment (with the 50 g/L solution in the tube) and calculate the concentration of salt that should be attained in the large container after long time. g. Sketch the concentration for the second case as a function of time for a long period. To do so correctly, estimate the time constant for this process.
Diffusion of salt
Click to obtain pdf for printing Data taken from an experiment published by Vernier, Biology with computers, Experiment 4. |
