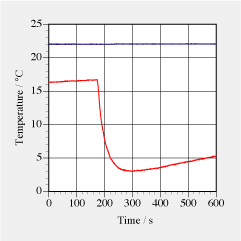
Sodium bicarbonate (NaHCO3) reacts with citric acid (H3C6H5O7) according to H3C6H5O7(aq) + 3 NaHCO3(s) ––> 3 CO2(g) + 3 H2O(l) + Na3C6H5O7 (aq) An aqueous solution of citric acid was prepared: 27.75 g of H3C6H5O7 in 99 g of H2O. 31.64 g of the solution was put in a small glass container to which 9.36 g of sodium bicarbonate (NaHCO3) were added. The citric acid solution had an initial temperature of about 16°C in an environment at 22°C (see graph of data on the right). Upon addition of bi-carbonate, the solution fizzes strongly (CO2 is produced and emitted) and becomes very cold. a. How much citric acid and sodium bicarbonate were used up (assuming that in perfectly balanced amounts all citric acid and all sodium bicarbonate would be used up)? b. How much CO2 should have been produced? c. Use the temperature data of the solution to estimate how much entropy flowed from the environment into the solution during the reaction. Assume the solution to have thermal properties of water. d. Taking the same data, what is the change of entropy of the solution during the reaction? e. CO2 has a molar entropy of 214 J/(K·mole). How much entropy must have been carried away by the CO2 produced in the reaction? | |
Click to obtain pdf for printing f. With the results obtained, estimate the amount of entropy produced during the reaction. g. Use the results calculated so far to estimate the amount of energy released in the chemical reaction. Use this to determine the chemical potential difference of the reaction. h. Discuss the uncertainties in the results obtained. What additional measurements would make your results more accurate? |
