1. Amount of Substance | |

| |
Amount of substance Chemical processes depend upon the amount of "stuff" of different chemical species. The amount of substance is not equal to mass or to volume (two quantities which are often taken as amount of "stuff" or "matter" in everyday life). For a single species, there is a simple relation between these three measures: 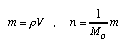 4.1 4.1 n: amount of substance; m: mass; V: volume; rho: density; M_o: molar mass of species. Unit of amount of substance: [n] = mole. The amount of substance measures how much of a species is present in a chemical sense (number of particles; 1 mole = 6.022·10^23 particles). For example, if sodium and chlorine are both completely consumed in a reaction that yields NaCl (table salt), the there were equal amounts of substance of Na and Cl present. If a certain amount of hydrogen has is used up completely with oxygen gas—which is also consumed all the way in forming water—then n_H2 = 2·n_O2. | |
Amount of substance in simple gases The measure of "stuff" appearing in the equation of state of the ideal gas is the amount of substance: At standard conditions (T = 298.15 K = 25°C, p = 1.013 bar), one mole of an ideal gas has a volume of 24.5 Liters (independent of the type of chemical!). R = 8.314 J/(mole·K) is the universal gas constant. | |
Processes leading to changes of amount of substance Chemicals (their amount of substance) can be changed as a consequence of two types of processes:
1. Chemical reactions that produce or consume some species. 2. Conductive transport of a species into or out of a storage area. 3. Convective transport of a species into or out of a storage area. | |
The law of balance of amount of substance The amount n of substance of a species in an area of space (or in a container) changes at a rate that is determined by the sum of all process quantities:
| dn/dt = I_n_net, cond + I_n_net,conv + Pi_n,net | 4.3 | | |
Stoichiometry of a chemical reaction In a chemical transformation (reactions and transports) where all species are completely consumed, the amounts of the participating species are integer multiples of a basic amount: A1, A2… are reactants, B1, B2… are products. The stoichiometric coefficients carry values such as 1,2,3… or 1/2, 1/3… | |
Molar quantities Quantities that are stored in materials or that are otherwise substancelike—such as entropy, mass, charge, volume—can be referred to the amount of substance of the body. We use small letters with an overstrike to denote the molar quantities. Here are molar entropy and molar volume: 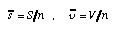 4.4 4.4
The well known molar mass is defined analogously: 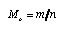 4.5 4.5
| |
Densities and concentrations Concentration measure are used to express indirectly amounts of substance of a particular species—the solute—dissolved in a solvent. There are many different measures called concentration. Mass fraction. This is defined as the mass of the solute (s) of a species divided by the total mass of the solution (solvent f and solutes s):
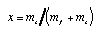 4.6 4.6
Molar (or mole) fraction. The ratio of the amount of substance of a species i and the total amount of substance of the solution: 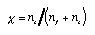 4.7 4.7
Mass-volume fraction. This is the ratio of the mass of the solute and the volume of the total solution (even though it looks suspiciously like the standard density, it should not be confused with this concept):
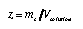 4.8 4.8
Molar concentration (molarity, unit M = mole/liter). This is the standard measure of concentration used in much of the following. It is defined as the amount of substance (dissolved) divided by the total volume of the solution:
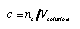 4.9 4.9
Molality and molinity. These terms denote the ratio of amount of substance to the mass of the solvent (molality) or the total mass of the solution (molinity). | |