2. Chemical Driving Force and Chemical Potential | |

| |
Driving force of chemical conversion Chemical conversion is the transport, production or desctruction of substances. We make a chemical driving force responsible for such conversions. Intuitively, the driving force can be understood as a "tension" between speicies in the original and final states associated with the conversion. The chemical driving force is defined as the difference of the chemical potentials of the species undergoing conversion in their initial and final states. See below. Examples: Reaction of hydrogen gas and oxygen gas to form water. There is a "tension" between water and the educts. In a dry climate, water evaporates from a puddle. There is a "tension" between the water in the liquid form and the (little bit of) vapor in the dry air. When the air is very humid (saturated), the evaporation stops (the driving force has become zero). | |
Chemical potential A value of chemical potential is associated with every substance. This value can be imaginged to be the measure of the tendency of the substance to change (to migrate or to decay). In a conversion, the difference of the (sum of) potentials of participating substances in their initial and final states is said to the the driving force of the conversion. Symbol: mu. Unit: [mu] = Gibbs (1 G = 1 J/mole) The chemical potentials of the elements in their most stable configurations are given a value of 0 Gibbs. | |
Calculating the driving force of a chemical conversion The equation of a general conversion can be written as 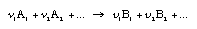 4.10 4.10
A1, A2… are reactants, B1, B2… are products. The difference of the chemical potentials of the conversion, [delta_mu]R, is calculated as follows: 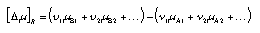 4.11 4.11 | |
Voluntary and involuntary conversions Chemical reactions and transports run by themselves from higher to lower chemical potentials. Therefore
1. [delta_mu]R < 0: Voluntary conversion 2. [delta_mu]R > 0: Involuntary conversion | |
Chemical equilibrium When the driving force of a chemical conversion vanishes, so does the conversion. Chemical equilibrium therefore requires the difference of chemical potentials to be equal to zero:
Equilibrium: [delta_mu]R = 0 | |
Dependence of chemical potentials on other factors Chemical potentials of substances depends upon many factors such as temperature (steam condenses at room temperature), concentration (water migrates from a piece of fresh bread to a dry piece in the same bag), pressure (water flows from points of high to low pressure, chemical reactions are influenced by pressure changes). | |
Dependence of the chemical potential upon temperature If changes are not too great, one can always approximate the dependence of the chemical potential by a linear expression: 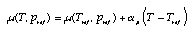 4.12 4.12
ref refers to a reference state. It can be proved that the temperature coefficient am is equal to the negative molar entropy (entropy per amount of substance) of the substance: 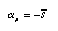 4.13 4.13 | |
Dependence of the chemical potential upon pressure As in the case of temperature, the chemical potential can be approximated by a linear relation: 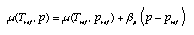 4.14 4.14
The pressure coefficient beta_m of the chemical potential is equal to the molar volume of the substance: 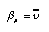 4.15 4.15 For incompressible fluids such as water we therefore have 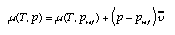 4.16 4.16 In the case of the ideal gas, the chemical potential depends upon the pressure as follows (this follows from gravito-chemical equilibrium with the pressure as a function of height in an isothermal atmosphere): 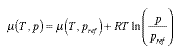 4.17 4.17 | |
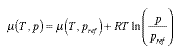 4.17
4.17