7. Dynamics of Chemical Reactions | | 
| | Relations between production rates in chemical reactions A chemical reaction such as in Equ. 4.33 can run in both directions, depending upon which side of the final equilibrium conditions the reactants and products are on. When the chemical driving force is positive, the reaction is assumed to go from left to right: 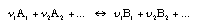 4.33 4.33 There is a production (destruction) rate for every species taking part in the reaction. These are not independent. In fact, there is a single reaction rate which can be taken to be one of the production rates. All the other rates depend upon this single rate: 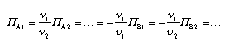 4.34 4.34
| | Reaction rates for simple reactions The expressions for production or reaction rates are derived just as the flow of a species from one environment into another was determined (Section 6). For a simple reaction A <–> B (such as the decay of a-glucose) we have: 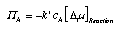 4.35 4.35
which, when linearized and introduced in the equation of balance, yields 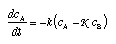 4.36 4.36
A chemical reaction involving two species on either side of the reaction such as the decay of murexide, i.e., A + B <–> C + D , leads to 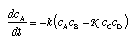 4.37 4.37
K is the equilibrium constant for the reaction, and k depends upon circumstances.
| | Orders of reactions It is important to note that Equ. 4.36 and Equ. 4.37 are two of the simplest possible results for the form of reaction equations. As long as reactions lead to equilibria of the form derived in Equ. 4.27 (which itself is just a simple example of its kind), we can expect reaction equations like the ones derived here. The reaction of Equ. 4.36 is said to be of 1st order, that of Equ. 4.37 is a 2nd oder reaction. In general, however, reactions are much more complex. Often, reactions proceed in several steps from the reactants to the products, where the intermediate reactions might not be known. Therefore, reaction rates often involve powers of the concentrations of the species taking part in the reaction. These powers, along with the reaction constants k, have to be determined by comparing models and experimental data. | | | |