Our atmosphere is a mixture of different gases, mostly nitrogen and oxygen. In the table, the mass fractions of the most important compon- ents are given. In the graphs, the concnetration of CO2 in ppmv is shown as a function of time.
a. Determine or find the molar masses of the substances shown in the table. Determine the molar mass of the mixture of gases in the atmosphere. b. Determine the composition by volume (volume fraction) of the components shown. Compare your result for CO2 with the data shown in the diagrams. c. Determine the molar fractions of the gases shown in the table. d. Take 1.0 m^3 of dry air at a pressure of 0.97 bar and a temperature of 20°C. What are the partial pressures of the components listed in the table? Does your answer depend upon how much air you take? e. Calculate the mass of the atmosphere of our planet. Hint: Calculate the weight of the atmosphere by using the atmospheric pressure at sea level and and surface area of the planet. You should get a value close to 5.3·10^18 kg. f. Find the amounts of substance for the gases listed in the table. In particular, find the amount and the mass of CO2 in the atmosphere. g. How much CO2 was in the atmosphere in 1750 and in 2005? According to the data, what has been the rate of change of amount of substance and of mass of CO2 in the air? h. The graph on the left shows seasonal | | Composition of dry air
Gas |
| x_m | Nitrogen | N2 | 0.7552 | Oxygen | O2 | 0.2315 | Argon | A | 0.0128 | Carbon Dioxide | CO2 | 0.00046 | Neon | Ne | 0.00012 |
CO2 concentration 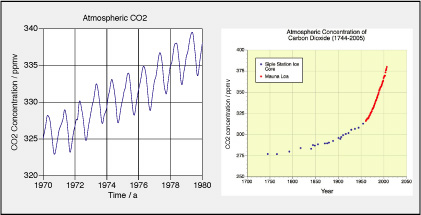
Click to obtain pdf for printing
variations of CO2 in the northern hemisphere. How much CO2 is bound by the growth of biomass each year? i. We read that in 2005 the rate of production ofcarbon (not CO2) from burning of fossil fuels was 8000 million tons per year. How does this compare to the rate of increase of CO2 in the atmosphere? j. What happens with the concentration of CO2 in the oceans if the concentration increases in the atmosphere? Why? |
