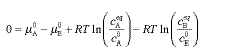CHAPTER 4 > READINGS > A BRIEF TEXT 5
5. Chemical Potential of Dissolved Substances | | ||||
| | |||||
Dependence of the chemical potential upon concentrationIn the case of solutions that are not too strong, the (partial) pressure of the dissolved substance in the solution obeys the equation of state of the ideal gas.
The pressure of the solvent (such as water) therefore is
Therefore, Equ. 4.17 holds as well for the pressure ps of a solute. Since the concentration of the solute is proportional to the pressure, the same form must hold for the concentration dependence of the dissolved substance (Fig. 1): | |||||
OsmosisThe pressure of the solvent (such as water) decreases according to Equ. 4.23b if the concentration of a solute increases. This effect is at the root of the phenomenon of osmosis (water or some other solute will flow from places where the concentration of a solute is lower to one where the concentration is higher). | | ||||
Chemical equilibrium of dissolved substancesChemical equilibrium is attained if | | ||||
Mass actionTraditionally, Equ. 4.27 is called the principle of mass action. |
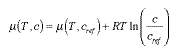 4.24
4.24